Santiago J. Molina (he/they) is a Postdoctoral Fellow at Northwestern University, with a joint appointment in the Department of Sociology and the Science in Human Culture program. They received a PhD in Sociology from the University of California, Berkeley in 2021 and a BA from the University of Chicago. Their work sits at the intersections of science and technology studies, political sociology, sociology of racial and ethnic relations, and bioethics. On a theoretical level, Santiago’s work concerns the deeply entangled relationship between the production of knowledge and the production of social order. Their research included fieldwork at conferences and in labs around the Bay Area.
In this visual interview, Julia Sizek, Matrix Content Curator and a recent PhD graduate in Anthropology from UC Berkeley, interviewed Molina about their research on CRISPR, the genetic engineering technology that has reshaped biological research through making gene editing easier. This new tool has excited biologists at the same time that it has worried ethicists, but Molina’s research shows how CRISPR has become institutionalized — that is, how CRISPR has become an everyday part of scientific practice.
This image depicts a model of the CRISPR-Cas9 system. How did you come to encounter this model of CRISPR, and how does CRISPR work?
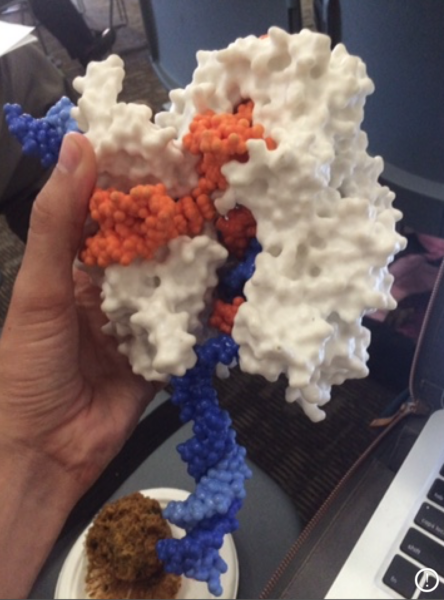
This model was passed around the audience at a bioethics conference in Davis, California back in 2014 when I started my fieldwork. I remember the speaker holding it high above his head and pronouncing, “This! This is what everyone is so excited about!” While he meant it as a way to demystify the new genome-editing technology, a 3D-printed model of a molecule doesn’t tell us a lot about the process behind the technology.
What is a bit disorienting is that technically, this isn’t a model of CRISPR at all, but a model of Cas9 (CRISPR-associated protein 9, a kind of enzyme called a nuclease) in white, an orange guide RNA, and a blue DNA molecule. To put it really simply, CRISPR (Clustered-regularly-interspaced-palindromic-repeats) describes a region of DNA in bacteria where the molecular “signatures” of viruses are stored so that the bacteria can defend itself. This bacterial immune system was repurposed by scientists into a biotechnology. At its core, CRISPR-Cas9 technology is just the white and orange parts. The Cas9 does the heavy lifting of cutting DNA, and the guide RNA, or gRNA, acts as the set of instructions that the Cas9 uses to find the specific sequence of DNA where it should cut.
While people use CRISPR as a shorthand for the entire CRISPR-Cas9 system, you won’t actually find a single Eppendorf tube in a lab marked “CRISPR.” As a process, the way scientists get this to work is by adding Cas9 and the “programmed” gRNA to cells via one of several delivery techniques, such as a plasmid or viral vector, so that the Cas9 will make a specific DNA cut. In the years since then, scientists have developed a whole toolbox of different Cas proteins, and each can make many different kinds of modifications.
What is interesting about this sociologically is that CRISPR has a wide scope of potential application, and early in its development, every possible use was on the table, from bringing back the wooly mammoth to ending world hunger. This meant that exactly what it would be, ontologically, was really open. Scientists would describe the technology as a pair of scissors, as a scalpel, as a find-and-replace function for DNA, a guided missile, a sledgehammer, etc. I became obsessed with these metaphors because they were traces of the active construction of CRISPR as a technology.
My research takes this focus on the development of genome editing technology and reframes it as a problem of institutionalization, which sociologists generally understand as the process by which a practice acquires permanence and reproducibility in society. I look at how the ideas around what the technology is, how it should be used, and what it should be used for come to be settled, legitimized, and eventually taken for granted.
CRISPR has recently been in the news, not only because of Emmanuelle Charpentier and Jennifer A. Doudna’s 2020 Nobel Prize, but because of the 2018 announcement that a Chinese researcher had used CRISPR to gene-edit babies. How has the media covered CRISPR and the ethics of the technology?

Most media articles go something like this: “The idea that scientists can modify your DNA at will sounds like science fiction. But now it’s reality!”
This framing does important work to normalize futures that are in active construction. When newspapers and magazines cover CRISPR, they are bridging the social worlds of science and civil society and making concrete a very fluid social process of knowledge production and technological development. In doing so, some media coverage amplifies the hype around CRISPR and genome editing.
That said, it’s more complicated than saying they sensationalize it, because most coverage draws directly from interviews with actual genome-editing scientists, and they do their best to represent the science accurately. Instead, I think about media coverage as part of the cultural side of institutionalization. News articles offer interpretive scripts though framing that audiences can use to make sense of what CRISPR is, how it is used, and what the ethical issues are. This “making sense” is part of how genome editing is coming to be seen as a normal practice in biomedicine.
The distinction between investigative reporting and general media is important to keep in mind. Take, for example, the controversy surrounding the birth of genetically modified twins in Shenzhen, China in November 2018. If it wasn’t for keen investigative reporting by Antonio Regalado of the MIT Technology Review ahead of the Hong Kong Summit, it is likely that the controversy would have unraveled differently.
The image above is a photo of a group of reporters during the summit taking pictures of He Jiankui, the scientist behind the clinical trial in Shenzhen that aimed to use CRISPR-Cas9 to confer genetic immunity to HIV in embryos. Subsequent media coverage of the controversy drew from interviews with high-profile, U.S.-based scientists in the field. These scientists argued that He Jiankui was an outsider on the fringe of the field. The resulting articles framed him as a “rogue,” “a mad scientist,” and a “Chinese Frankenstein.” This “bad actor” framing tells us that on the whole, the field is responsible and CRISPR itself is good, essentially repairing the crisis.
However, in alignment with more recent investigative reporting, my ethnographic research found that a handful of U.S.-based scientists had helped He Jiankui with his project. He had earned his PhD at Rice and was a postdoctoral fellow at Stanford. Scientists at UC Berkeley had given him technical advice on the project, as well. To me, this suggested that the “bad actor” framing — and the Orientalism surrounding how he was talked about – obfuscated the broader moral order of genome editing.
CRISPR is a relatively contemporary invention, but the idea of genome editing has a much longer history. How does this history appear in your research, and what does Charles Davenport have to do with it?

It’s interesting how little history appeared in my research. There is a sort of presentism that comes with “cutting-edge science.” CRISPR technology is part of a lineage of genetic engineering tools, going back to the 1970s, when recombinant DNA (rDNA) was invented. This biotechnology, rDNA, allowed scientists to mix the DNA of different organisms. It gave rise to a whole industry of using engineered bacteria to produce biologics and small molecules like insulin. The history of rDNA is important because the debates around its use in the 1970s came to be the dominant model of decision-making surrounding new technologies in the United States. Indeed, a handful of the top scientists from these debates have held top positions on committees that have been tasked with debating the ethics of genome editing over the past five years.
Charles Davenport predated these debates, and has been largely an invisible figure for modern genome-editing scientists. Davenport was a prominent scientist in the early 20th century. He was a eugenicist and racist scientist who served as the director of Cold Spring Harbor Laboratory, a private, non-profit research institution, from 1898-1924. While at CSHL, Davenport founded the Eugenics Record Office, which published research to support the eugenics movement. I found this photo of Davenport in Blackford Bar, the pub at Cold Spring Harbor Laboratory, where I went to the first meeting, titled “Genome Engineering: The CRISPR/Cas Revolution,” in 2015. While the scientific community eventually came to reject Davenport, and the eugenics movement fell out of fashion after World War II, this history is important to recognize as we usher in a new technology aimed at eliminating genetic diseases and improving human health. At the conference in 2015, I thought, if Davenport’s ghost had been hanging out at the pub, he would have been thrilled.
The scientists I worked with vehemently rejected the idea that what they were doing could be considered eugenics, or what one scientist called it, the “E-word.” But people often forget that the eugenics movement in the United States was both mainstream and progressive at the time. Eugenics laws were drafted and passed by Democratic legislators who aimed to address poverty by drawing on the most up-to-date science, medical knowledge, and expert opinion. When this history was brought up at modern conferences and meetings, it was either subtly discredited as fear-mongering or tucked into a panel at the end of the conference to entertain philosophical discussion.
Your research also contends with the way research is conducted between different laboratories, even when many of the plasmids (a kind of DNA molecule commonly used in CRISPR applications) and techniques that they use are proprietary. The shipping area in this image is how Addgene, what has been called “the Amazon of CRISPR,” sends reagents and plasmids used in scientific research to laboratories around the world, and manages many intellectual property issues. What is Addgene’s role in the scientific process?

While I was doing my research, there was a raging patent dispute between the University of California, Berkeley and the Broad Institute, where each institute claimed to have invented the technique for modifying mammalian cells with CRISPR. So the proprietary aspects of CRISPR were always in the background. But I think if it wasn’t for Addgene, these concerns would have really slowed down the spread of genome editing.
Addgene is a non-profit organization that operates as a mediator between the exchange of practices and biological materials between labs. What they do is manage a plasmid repository, a sort of technique library, and fulfill the requests for plasmids to send them to those labs. Because plasmids are central to many biological experiments, and are key for CRISPR-based techniques, scientists rely on the availability of these circular pieces of DNA as a key reagent. Since receiving its first CRISPR plasmid in 2012, Addgene now has over 8,000 different CRISPR plasmids in the repository, and has shared them over 140,000 times with laboratories across 75 different countries. They essentially took over the logistics of CRISPR distribution, moving biological materials from place to place. By doing it at a really low cost, this effectively contributed to what scientists described as the “democratization” of genome editing.
They also keep patent lawyers at universities happy with detailed record-keeping and by electronically managing material transfer agreements (MTAs), which sort out the proprietary issues, through a Universal Biological Material Transfer Agreement (UBMTA). This UBMTA relaxes the institutional constraints on the transfer of biological materials. Scientists love this because it reduces a lot of paperwork.
Last but not least, Addgene contributes to the institutionalization of CRISPR-Cas9 by producing guidelines and protocols that support the use of some of the plasmids. For example, Addgene was the first to develop a textbook for CRISPR. Their CRISPR 101 eBook has been downloaded more than 30,000 times, and their informative CRISPR blog posts had been visited over 500,000 times as of 2019. In these materials, detailed definitions of new genome editing techniques and terms of art are spelled out for curious adopters. Additionally, the scientific team at Addgene works with the scientists who are depositing plasmids to coproduce useful documentation to accompany the plasmids. Addgene does not share plasmids with for-profit organizations, but acts as an up-to-date clearing house and tracker of CRISPR innovations in academic and non-profit laboratories.
As part of your research, you spent time at different labs around the Bay Area to understand how CRISPR research has become an ordinary part of scientific research. Can you walk us through some of these images of lab life and what they show us about how CRISPR has become institutionalized?


The first image is of the atrium in one of the buildings I often found myself in for fieldwork. The huge sculpture of ribosomes on the side looks so abstract to me. A lot of these spaces required keycard entry, and for me, the emptiness of some of the spaces made them all the more isolating. I would have to get lost sometimes just to find the right room, where a small group of scientists were discussing the next big breakthrough or the next application of CRISPR-Cas9. The public-facing image of the field was really different from the behind-the-scenes shop-talk environments where I took notes. It was different because it wasn’t open to anybody, and you would need a lot of intellectual and cultural capital to enter those places.
The second picture, to me, represents the ordinary that is behind those barriers of access. Lab benches are workshops. They are shared spaces that are a lot like kitchens in a restaurant. Everything has its place, every tool is in its nook, you might find some remnants of an experiment in the fridge, or old reagents in the freezer. But you can tell there is some fun in the mix. The folks who are working at those benches are doing it because they love it. For these graduate students and postdocs, CRISPR-Cas9 was an exciting opportunity, something that would help them finish their PhD, or if they were an undergrad-volunteer, it was a key skill to move forward. Lab life a lot of times felt banal: scientists moving through their careers, with lots of failed experiments, meetings that could have been emails, day-to-day conflict with coworkers, late hours, etc. I wish people could see the contrast between the hype surrounding something like CRISPR-Cas9 and the on-the-ground struggles of scientists in the lab.
In these pictures below, you show a humorously decorated doorway that tells us a lot about how scientific work happens at a university. What does this tell us about who conducts science, and about equity issues within the lab?


This personification of the lab was interesting to me because it draws attention to those struggles I just mentioned. Of course the decoration is a lovely piece of satire, but scientific discoveries and breakthroughs are the products of years of labor. A lot of this work is done by unpaid undergraduate volunteers, graduate students who are often in precarious financial situations, and some paid research associates, and it is coordinated by postdoctoral fellows. Sometimes, because of the demands of experimental work, lab workers would have to come in in the middle of the night to feed cells, check on experiments, or manage instruments. In the lab I worked in, one research associate worked as a Lyft driver on the side because their salary wouldn’t cover their cost of living. While the hierarchies of labor are still very strong, some universities and labs, like the Innovative Genomics Institute at UC Berkeley, are now requiring that all undergraduate workers be paid. I think this is a step in the right direction, but there are still equity issues both between and within ranks of the lab.
This disparity is even more extreme when you consider how senior scientists and universities benefit from scientific labor. Social capital in the form of reputation and financial capital both accumulate as a result of this work. Partnerships between university laboratories and the biotech and pharma industries in particular have become commonplace in 21st-century biomedicine. Research examining these partnerships describes this as academic capitalism or neoliberal science. My research adds to this line of social scientific research that has traced this institutional shift, where academic organizations are increasingly adopting the practices and bureaucratic frameworks of for-profit organizations in industry. Those patent disputes I mentioned previously are a good example of this.
With CRISPR research, as with much other biological research, the institutionalization of scientific norms is essential to conducting scientific research. What does Michael Jackson have to do with that?

There are three proximate institutions of social control surrounding scientific work, in my view: biosafety, bioethics, and the ethics of research misconduct. This poster is an example of a biosafety rule being operationalized in the lab. It is posted on the doors so you would see it as you exit the lab space to the common area and kitchen. Biosafety essentially aims to contain the materials, reagents, and products of scientific experiments to the lab. Lab managers and principal investigators must fill out detailed forms describing the experiments being done and submit these to the biosafety office at their university. These are then reviewed and evaluated by biosafety experts, who then make recommendations about infrastructure requirements for the spaces where the experiments are conducted and prescribe mandatory training for any personnel conducting those experiments.
Biosafety is a really interesting social institution because it must constantly keep up with new techniques and develop risk frameworks for assessing them. For innovations like CRISPR-Cas9 that are revolutionary, this sometimes requires some finesse. When you consider the modifications being made to bacteria, plants, non-human animals, and human cells, you can bet there is considerable work going into making sure those biologics don’t end up where they aren’t supposed to. Consequently, scientists must follow strict protocols for waste disposal and use the appropriate personal protective equipment (PPE).
But then consider who is doing those experiments. There can sometimes be a disconnect between the official protocols and how they are enacted. This poster captures that disconnect and suggests that more immediate forms of social control might work better in some cases than extensive bureaucratic procedure. Plus, Michael is iconic.
As with any social process, there are bound to be accidents. In the lab I observed, for example, a graduate student accidentally cut himself through his gloves on some broken glass while conducting some genome-editing experiments with lentiviral packaged Cas9. This lentivirus could, in principle, infect any mammalian cell. While he was working under the fume hood, which creates negative pressure to suck up the air where the experiment is being done, there was still a risk that Cas9, which would edit the DNA, could enter his blood stream. He then went to the post-doc he was working under and the lab manager, who advised him to report it to the Office of Environment, Health & Safety (Eh&S). EH&S then told him to go to the student health center. Once at the health center, the grad student with his bandaged hand informed the nurse that his lab was categorized as BSL-3 (biosafety level 3), to which the nurse responded, “What is BSL-3?” He was ultimately fine, as far as we know, but the example shows a further disconnect between the different offices tasked with managing the risks of scientific work.
As genome editing continues to develop as a broader institution in biomedicine, there are going to be accidents, and there is going to be misuse. No number of guidelines or codified norms can prevent that. This is why it is crucial that we continue having debates about the norms governing the use of the CRISPR-Cas9 system, both as a promising clinical technique and as a sociocultural institution. My hope is that these debates will lead to concrete regulatory and legal changes that can more directly shape this technology’s use.



